


∗ Two (2) European-territory based manufacturing plants adhere to Rontis’ vertically integrated
processes, allow production flexibility and ability to comply with customer’s set specifications
∗ Vertical Production Philosophy (VPP) is applied to all production stages, including extrusion, injection
molding, assembling, packaging, testing, in-house ETO Sterilization (alternative types of sterilization
available upon request) and final product release, ensuring uninterrupted & sustainable supply chain
∗ Production of tubing, components and final product is totally in Clean Room Areas classified as:
ISO Class 8, GMP Grade C
∗ In-process quality controls in all stages of the production processes, including:
• Visual inspection,
• Dimensional control
• Functionality testing
• Leak testing
• Packaging/Packing control.
∗ In-house sterile final product testing including:
• Technological control of Functionality and Resistance
• LAL -Bacterial endotoxin testing
• Sterility testing
• Chemical testing
∗ Medical Grade bio-compatible raw materials sourced carefully from selected European suppliers and
compounded to achieve highest degree of performance, purity and quality
∗ Thermoforming Pouch Packaging with Peal Open corner
∗ GMP certified European-territory based manufacturing plants
∗ Available for OEM / co-labeling manufacturing
∗ Tubing and individual components’ supply available upon request
∗ Certified by DEKRA Certification GmbH for establishing and maintaining QMS according to EN ISO 13485
∗ EC certification according to Directive 93/42/EEC at is valid
∗Open to discuss special projects from design to validation & scale up to production



Rontis offers since 1999 a wide range of
Hemodialysis Blood Tubing sets, according
to international standards and available
in customized configurations to meet the
individual needs of its customers.
Rontis is able to develop and provide custommade bloodline circuits for hemodialysis
treatment that could be paired with most of the
existing dialysis machines in the market:
∗ Fresenius*
∗ Baxter
∗ Gambro
∗ Hospal
∗ B.Braun
∗ Bellco
∗ Nikkiso
∗ Nipro
∗ Drake Willock
- Universal Bloodlines
*Only for certain markets
HDF on-line sets
∗ Fresenius*
∗ B.Braun
∗ Drake Willock
∗ Nikkiso
*Only for certain market

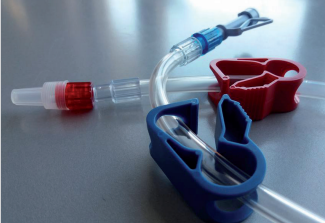
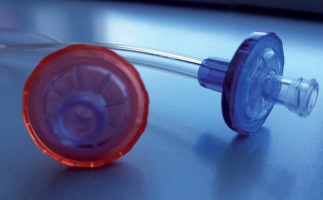
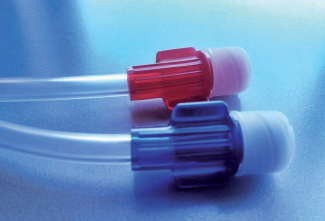
∗ Pump segments with superior material quality, to
maintain stable blood flow during the treatment and
blood turbulence within the tube
∗ Ergonomically designed universal dialyzer
connectors that ensure compatibility with all the
dialyzers in the market
∗ Color-coded connectors for easier identification
∗ Luer lock connections compatible with all branded
fistula needles female luer hubs, as well as shunts,
catheter and subclavian connections
∗ High quality tubing for continuous blood flow and
easy priming procedure
∗ Smooth inner surfaces for the reduction of foaming
and facilitation of air removal as well as minimizing the
hemolysis phenomenon
∗ Transducer protectors with transparent windows
∗ Injection ports with finger protection shields
∗ Latex-free injection sites for patient’s and healthcare
provider’s convenience
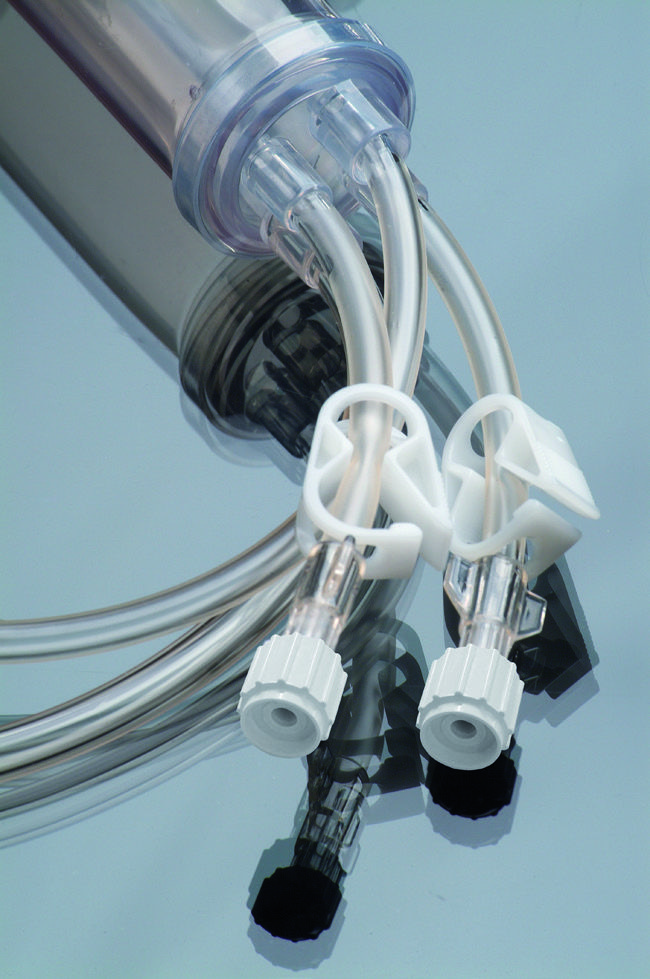
Product Description:
Sterile Y – type extension line, with two Female Luer
Lock connections and one Male Luer Lock connection
∗ Rotating Male Luer lock
∗ Crystal Clear Transparent Tube
∗ Tube outside diameter 16fr
(12fr available upon request)
∗ Length 17cm (additional sizes available upon request

Hemodialysis Device Disinfectant
Concentracted/ Liquid
5000 ml

∗CHEMICAL COMPOITION
50% Citric Acid, Monohydrate and auxiliary agents
∗PRODUCT SPECIFICATIONS
THERMO CLEAN 50% provides cleaning, decalcification and disinfection of organic and inorganic
waste that forms on the generator and lines of haemodialysis equipment during haemodialysis process.
It is compliant with stainless steel, plastic materials and ceramic surfaces. It should not contact with
plexiglass and aluminium surfaces.
∗INTENDED USE
ThermoCIean 50% is compliant with stainless steel, glass, plastic materials and ceramic surfaces. Do
not use it on sensitive surfaces such as plexiglass and aluminium that may react with acids.
* USAGE
ThermoCIean 50% is used by automatic vacuum through disinfection pipette Of the haemodialysis
machines. Observe recommendations of the machine manufacturer. pH=1-3
∗ANTIMICROBIAL CHARACTERISTICS
Bactericidal, Fungicidal, Virucidal, Micobactericidal,Tuberculocidal

Disinfectant wipe for surfaces
120 sheets

* CHEMICAL COMPOSITION
Benzalkonium chloride, Didecyldimethylammoniumchloride and excipients
* PRODUCT SPECIFICATIONS
Ready Wipe is ready-to-use and has an extended antimicrobial action spectrum with the quaternary
ammonium compounds in its composition. It does not contain aldehyde, phosphate and alcohol. It is
recommended to use on surfaces with metal parts since it contains corrosion inhibitor. Ready Wipe is
compatible with glass, ceramic, silicone, plastic (including Plexiglas), wood and stainless steel
materials.
* AREAS OF USE
It is used for the cleaning and disinfection of all horizontal and vertical surfaces and especially
incubators, PVC epoxy areas, floors, walls, ultrasonography probes, incubators, aerators and
micromotor tips used in dentistry, disinfection requiring medical devices and equipment in ENT units
as well as operating rooms, intense care rooms, in the hospitals that require quick disinfection.
∗USE
Ready Wipe, is a ready-to-use spray disinfectant suitable for use on all horizontal and vertical
surfaces. It is sprayed from a distance of 30cm-40cm . It takes 30 seconds for the effect to appear. It
is wiped with a clean cloth and allowed to dry.
∗ANTI-MICROBIAL CHARACTERISTICS
Bactericide, Fungicide, Virucide, Antimycobacterial, Tuberculocide

• Connection & disconnection sets for hemodialysis
fistula and catheter therapy.
• Nova Dialysis Sets are customised for specific needs
and practises with safe and easy way.
• Sterile.



Dedicated to patient well-being, operational excellence, and global collaboration to deliver exceptional healthcare solutions.
© 2025. All Rights Reserved – PRIMEDIC